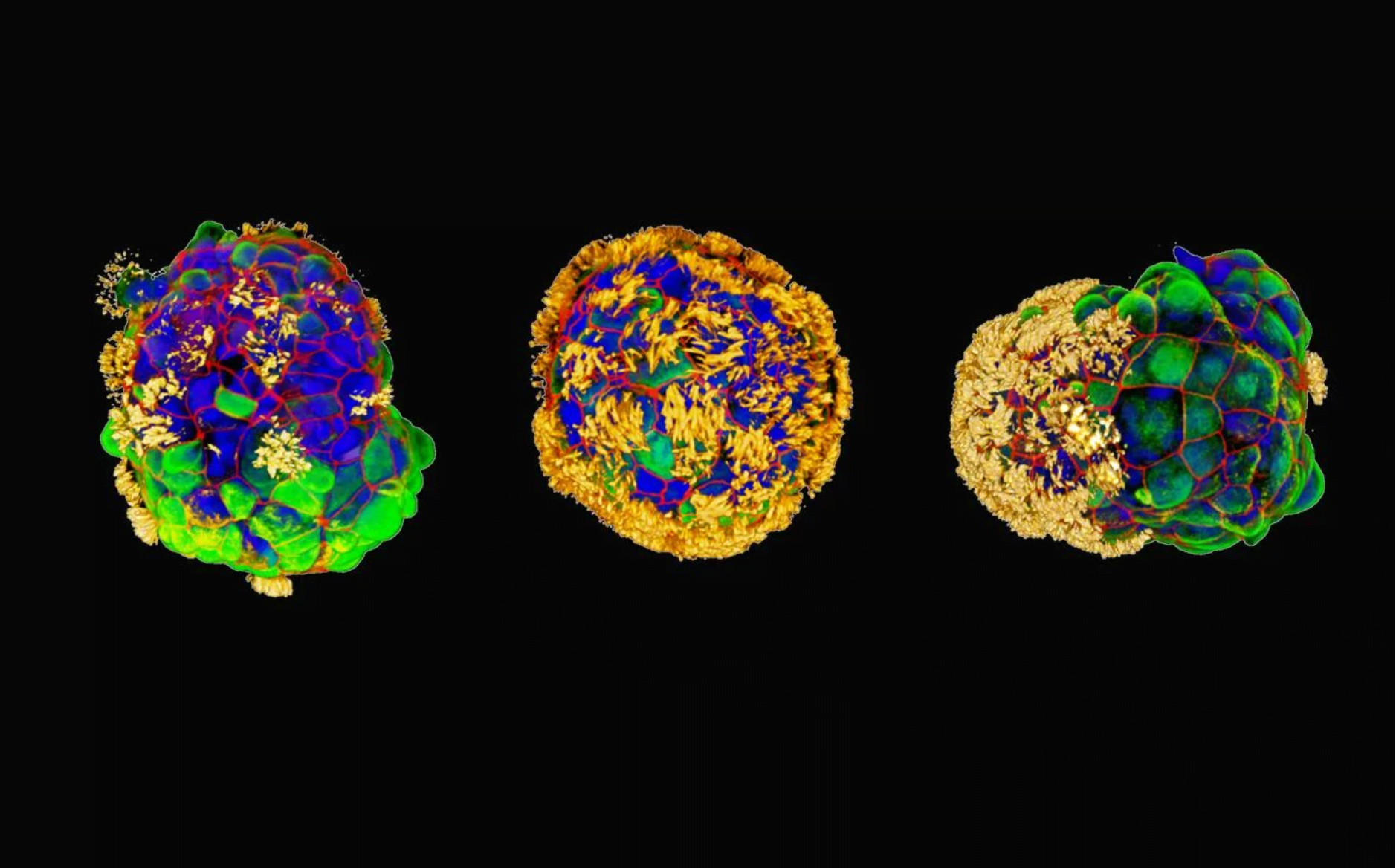
Researchers at Tufts University have recently unveiled a groundbreaking development in the field of biobots—tiny biological robots called Anthrobots. These multicellular bots, crafted from human tracheal cells, have demonstrated the ability to not only move across surfaces but also facilitate the growth of neurons, showcasing healing effects in laboratory experiments.
Unlike their amphibian-derived predecessors, the Xenobots, Anthrobots are constructed from adult human cells without any genetic modification. Ranging in size from the width of a human hair to the point of a sharpened pencil, these self-assembling bots have shown promising therapeutic potential. The study, led by Professor Michael Levin and Ph.D. student Gizem Gumuskaya, aims to explore the broader question of how cells can be recombined into different “body plans” to carry out diverse functions by design.
In their experiments, the researchers observed that Anthrobots could not only create new multicellular shapes but also move across a surface of human neurons in a lab dish. Remarkably, they encouraged the growth of neurons to fill in gaps caused by damage, showcasing their unexpected healing capabilities. The researchers referred to a clustered assembly of Anthrobots as a “superbot,” under which neurons grew, though the exact mechanism remains to be fully understood.
One notable advantage of using human cells is the potential to construct these bots from a patient’s own cells, minimizing the risk of triggering immune responses. Anthrobots, lasting only a few weeks before naturally breaking down, can be easily re-absorbed into the body after completing their therapeutic tasks. Furthermore, the Anthrobots can only survive in specific laboratory conditions, eliminating the risk of unintended exposure or spread outside the lab.
The study also revealed the fascinating process of Anthrobot creation. Starting as a single cell derived from an adult donor’s trachea, these cells, covered with cilia, were encouraged to form multicellular spheres called organoids. The Anthrobots exhibited diverse shapes and movements, presenting a unique biorobotics platform that fills the gap between nanotechnology and larger engineered devices.
Looking ahead, the researchers envision a range of therapeutic applications for Anthrobots, including clearing plaque buildup in arteries, repairing spinal cord or retinal nerve damage, recognizing bacteria or cancer cells, and delivering targeted drug treatments. The ability of Anthrobots to encourage neural growth without genetic modifications suggests a potential for efficient healing in various tissues.
Anthrobots represent a leap in the field of biobots, possibly opening doors to innovative therapeutic tools for regeneration and disease treatment. As researchers delve deeper into understanding the healing mechanisms and exploring additional capabilities of these Anthrobots, the future holds exciting possibilities for the intersection of biology and robotics.