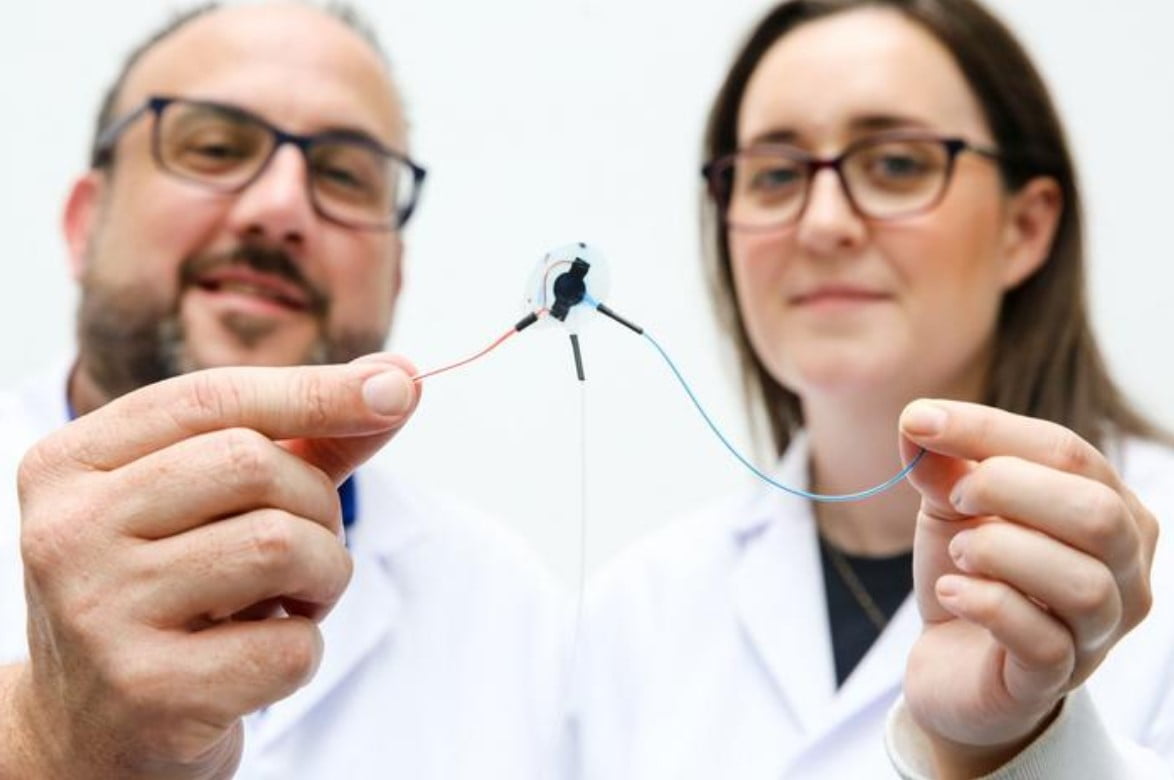
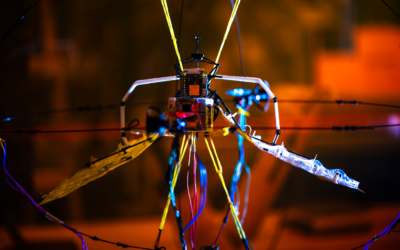
In the realm of medical device implants, scar tissue, also referred to as fibrosis, has long been a significant challenge. Patients undergoing potentially life-saving treatments often encounter issues with scarring around foreign objects, leading to malfunctions and failures of the implants. However, a groundbreaking discovery merging soft robotics and artificial intelligence (AI) could soon revolutionize this landscape.
A recent report published in Science Robotics details a collaborative effort between researchers from MIT and the University of Galway, resulting in a novel medical device technology that leverages AI and a flexible structure to counteract scar tissue formation.
Co-lead author and postdoctoral candidate at the University of Galway, Rachel Beatty, envisions a future where therapeutic implants possess the capability to sense their environment and respond using AI. This paradigm shift holds the potential to reshape implantable drug delivery for chronic diseases, marking a pivotal advancement in medical science.
At the core of this innovation lies a conductive, porous membrane that detects the onset of scar tissue blockage. When this signal is detected, a machine learning algorithm takes charge, implementing a process called mechanotherapy. During mechanotherapy, soft robotic implants inflate and deflate at varying speeds and sizes to prevent the formation of scar tissue.
MIT Professor of Mechanical Engineering and co-author of the study, Ellen Roche, highlights the potential of personalized drug delivery systems that adapt to individual immune responses. These devices have the potential to minimize off-target effects and ensure precise drug dosages at optimal times.
Through training simulations, the researchers’ device exhibited the ability to create personalized, consistent dosage plans in scenarios involving extensive fibrosis. Even in challenging situations characterized by thick and dense scar tissue, the AI-driven device showcased effective control over drug release.
Garry Duffy, senior author of the study and Professor of Anatomy and Regenerative Medicine at the University of Galway, shares the initial focus of using this technology for diabetes treatment. Duffy emphasizes the potential to extend the lifespan of insulin delivery cannulas, thereby reducing the frequency of replacements and enhancing the experience of individuals managing diabetes.
Looking beyond diabetes treatment, the researchers envision a future where this technology can be adapted for various medical scenarios and drug delivery schedules. Duffy anticipates that these advancements could establish consistent and responsive dosing over extended periods, reducing the need for clinician intervention and minimizing device replacement due to scar tissue-related issues.